21 CFR Part 11 Workflows in LabImage
The following list shows a typical workflow for a 21 CFR Part 11 compliant installation including the process to aquire image data from an external device.
| Workflow Steps | Description | Screens and Image |
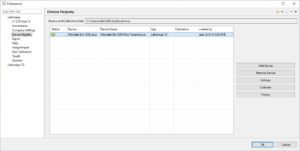
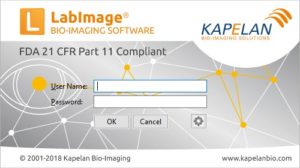
| Login into LabImage | User name and passwort is used to login into LabImage. LabImage allows definition of:
|  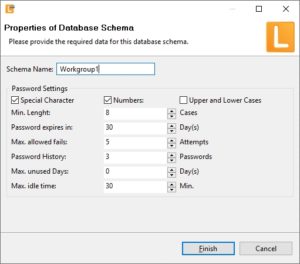 |
| Create new analysis using imagers | Imagers (e.g. Bio-5000 scanner) can be used to generate image within a closed system. Image data will never leave that system so data integrity is guaranteed.
Learn more about how to integrate Microtek Bio-5000 2 platform scanner using methodes, | |
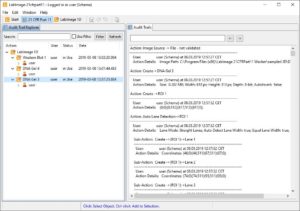
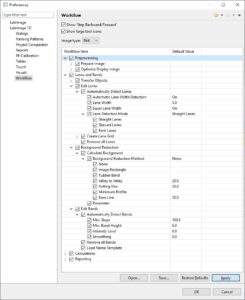
| Analyze your data | Data Analysis is supported by LabImage’s workflow based system. Each workflow can be customized to specific needs.
| |
| Approve data | After completion projects can be approved. Approve depends on user rights.
| 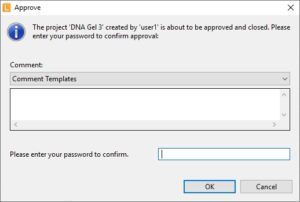 |
| Summary | LabImage provides a closed workflow to generate images, analyze and document data according to FDA 21 CFR Part 11.
|  |